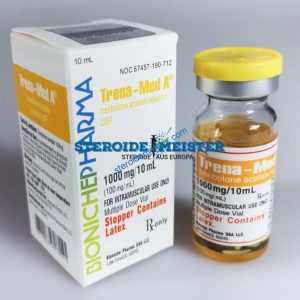
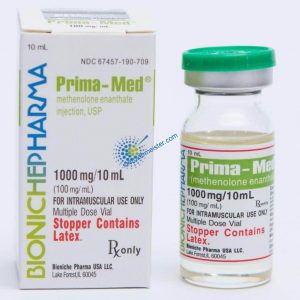
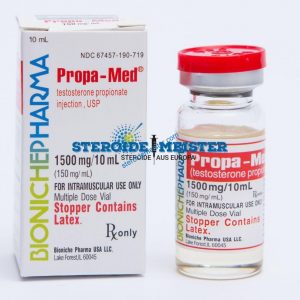
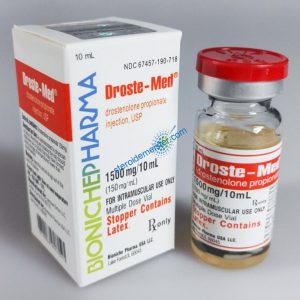
Angebote des Tages
Gehe zu Tägliche Angebote SektionSteroideMeister® – Steroide sicher online kaufen!
WER WIR SIND
In der Bodybuilding-Welt sind anabole Steroide oder einfach „Steroide“ weit verbreitet, da sie Bodybuildern dabei helfen, das Muskelwachstum schnell zu fördern. Viele Menschen auf der ganzen Welt verwenden Steroide als Chemikalie für die sofortige Muskelentwicklung und um den Appetit zu fördern, das Knochenwachstum zu stimulieren und die Auswirkungen von Muskelschwund aufgrund chronischer Krankheiten wie Krebs und AIDS zu verringern.
Aber Steroide zu kaufen ist eine schwierige Aufgabe für alle. „Wie kaufe ich Steroide?“ und „Woher bekomme ich Steroide?“ Sind die am häufigsten gestellten Fragen. SteroideMeister.com ist möglicherweise die beste Steroidquelle für Sie, mit der Sie die gewünschten Steroide direkt von Ihrem Computer aus kaufen können. Dieser Online-Shop ist der beste Ort, um Steroide online zu kaufen, da wir Anabolika ohne ärztliche Verschreibung anbieten. Vielmehr bieten wir eine schnelle und einfache Lieferung von Steroiden zu einem sehr günstigen Preis. Steroide werden nicht nur von Bodybuildern verwendet, sondern auch, um fit und gesund zu bleiben.
WARUM WIR?
In unserem Online-Shop können alle Kunden, einschließlich Bodybuilder, Sportler, Patienten usw., auf einfache Weise Steroide von höchster pharmazeutischer Qualität erhalten. Diese Steroide werden in einer lizenzierten Umgebung unter strenger Aufsicht hergestellt. Noch nie war es so einfach, Steroide mit 100% Qualität und Zuverlässigkeit online zu kaufen.
Bei SteroideMeister.com erleben Sie das beste Steroid-Einkaufserlebnis, da wir eine garantierte Lieferung, einen 24-Stunden-Kundendienst, keinen Mindestbestellwert, eine SSL-gesicherte Zahlung und eine garantierte Diskretion anbieten und auch keine Verschreibung verlangen. Wir sind ein zuverlässiger Online-Steroid-Shop und bieten Steroide aus Europa, Indien, Asien, Griechenland, der Türkei, Großbritannien usw. an.
Originale und echte Steroide kaufen
Vor dem Kauf von Steroiden gibt es verschiedene Dinge zu betrachten. Steroide, die von legalen Chemikern verkauft werden, helfen Ihnen nicht nur dabei, die Masse zu erhöhen, sondern halten Ihren Körper auch von Nebenwirkungen fern. Wir sind stolz darauf, dass eine riesige Auswahl an Produkten von seriösen Pharmaunternehmen hergestellt wird. Bei anderen Quellen können Sie nicht sicher sein, was Sie injizieren. Sie können kontaminiert sein, Schadstoffe, andere als die angegebenen Steroide oder in einer anderen Dosis oder überhaupt kein Steroid enthalten. Mit SteroideMeister.com können Sie sicher sein, dass es sich bei der verabreichten Dosis um das eigentliche Produkt handelt. Sterilität, Inhalt und Qualität des Produkts sind garantiert. Die bei SteroideMeister.com erhältlichen Steroide erfüllen alle Ihre anabolen Anforderungen.
Warum hier einkaufen?
Anabolika Blog
Bewertung: 4.83. Basierend auf 1940 Bewertungen. Klicken Sie hier, um unsere Bewertungen zu überprüfen